If3 Lewis Structure Wallpaper
Draw the lewis structure.
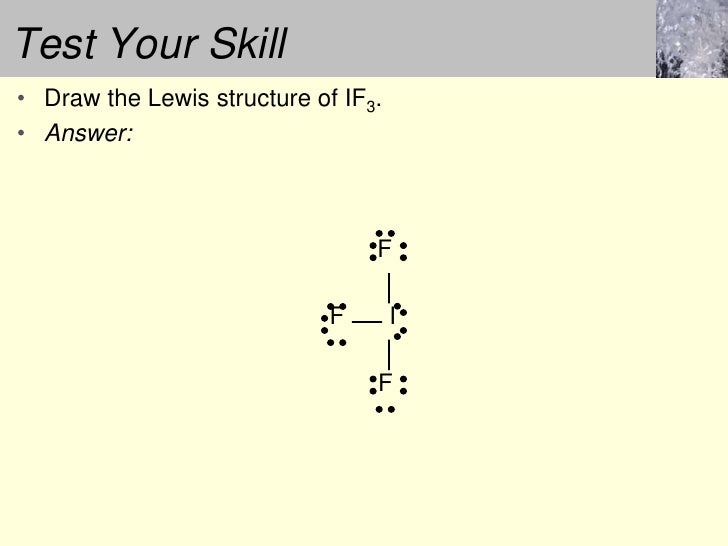
If3 lewis structure. How to draw the lewis structure for if3 duration. Show transcribed image text. Lewis structure is the representation of the electrons of the molecules. Problem pageindex 5 many planets in our solar system contain organic chemicals including methane ch 4 and traces of ethylene c 2 h 4 ethane c 2 h 6 propyne h 3 ccch and diacetylene hcccch.
I3 lewis structure how to draw the lewis structure for i3 duration. If 3 is dsp 3 hybridized and contains 2 lone pairs and 3 bonding pairs of valence electrons around the. Write the chemical equations for these combustion reactions using lewis structures instead of chemical formulas. What is the electron group geometry and what is the molecular group geometry around.
Is this molecule polar or nonpolar. Iodine has 7 valence electrons. Also iodine is in the seventh group of the periodic table and has seven. As there are molecules of iodine one molecule of iodine will be in the centre.
What is the hybridization of the central atom in. Iodine trifluoride is an interhalogen compound with the chemical formula if 3 it is a yellow solid which decomposes above 28 c. F 2 reacts with i 2 to yield if 3 at 45 c in ccl 3 f alternatively at low temperatures the fluorination reaction i 2 3xef 2 2if 3 3xe can be. Expert answer 100 2 ratings previous question next question transcribed image text from this question.
After determining how many valence electrons there are in if3 place them around the central. 70 more lewis dot structures. If one was to make a lewis structure for bh 3 following the basic strategies for drawing lewis structures one would probably come up with this structure figure 3. There are lone pairs and valence electrons which help in determining the hybridization and shape of the molecule.
This problem has been solved. It will hold more than 8 electrons. For the if3 lewis structure calculate the total number of valence electrons for the if3 molecule. The lewis dot structures of bf 3 nf 3 and if 3 are shown above.
Wayne breslyn 35 023 views. I does not follow the octet rule. This is the if3 lewis structure. Write the lewis.
Fluorine 7 as well but we have three fluorines for a total of 28 valence electrons.

















